Medical Devices & MedTech Products
The U.S. Food and Drug Administration (FDA) has established classifications for more than 1,700 different types of medical devices.
FDA Center for Devices and Radiological Health (CDRH) is the part of FDA responsible for regulating organizations who manufacture, repackage, relabel, and import medical devices. In addition, CDRH regulates radiation-emitting electronic products (both medical and non-medical) such as lasers, x-ray systems, ultrasound equipment, microwave ovens and color televisions.
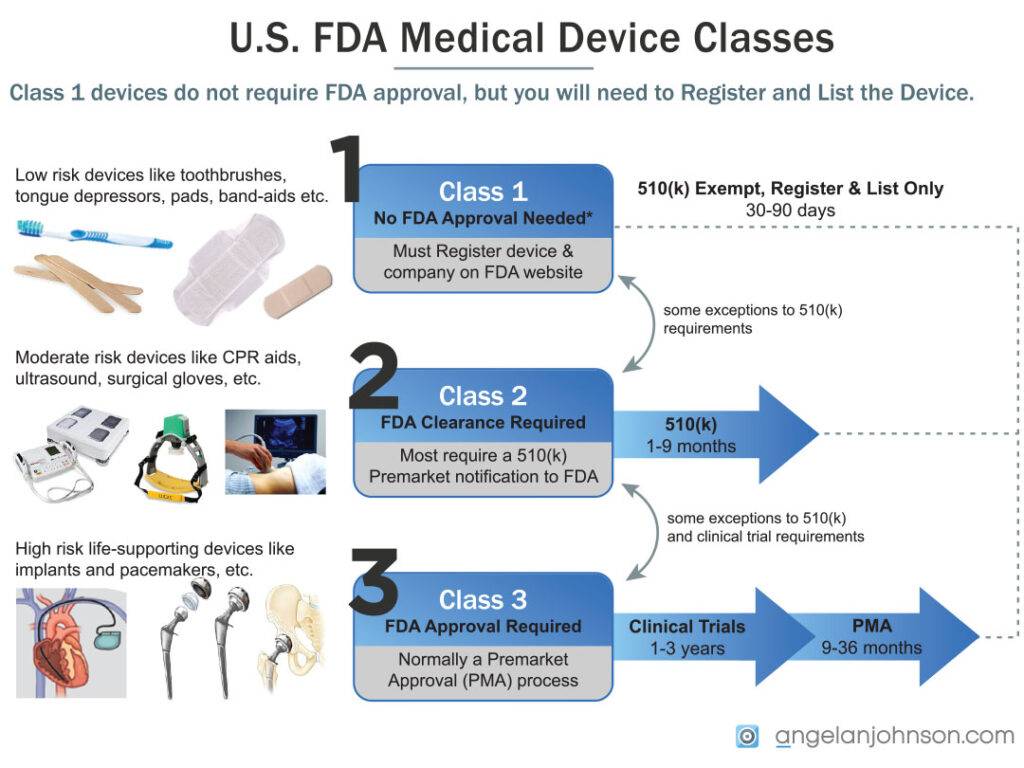
Each of these generic types of devices is assigned a medical device regulatory “class” one through three based on what FDA required to ensure the device is safe and effective.
Device Class and Regulatory Controls:
- Class I General Controls – Most required “Establishment Registration” and “Device Listing” and no separate filing with FDA
- Class II General Controls and Special Controls – These devices are similar to others already sold in the U.S. and typically required a 510(k) to be filed with FDA, with exceptions for certain products.
- Class III General Controls and Premarket Approval – These are new technologies with high risk, like implants, that require a PMA/HUD to be filed with FDA, and typically require extensive clinical testing.
The Federal Food Drug & Cosmetic (FD&C) Act defines medical device as an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including any component, part, or accessory, which is
(1) recognized in the official National Formulary, or the United States Pharmacopeia, or any supplement to them,
(2) intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or
(3) intended to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of its primary intended purposes.
Medical Device Services:
- Medical Device Establishment Registration & Listing Service
- 510(k) preparation
- Engineering and testing review
- Clinical Trial Advice (protocol development & Q-Sub FDA Meeting Support)
Related Services:
- EPA Disinfectant Registration
- FDA OTC Drug Registration
