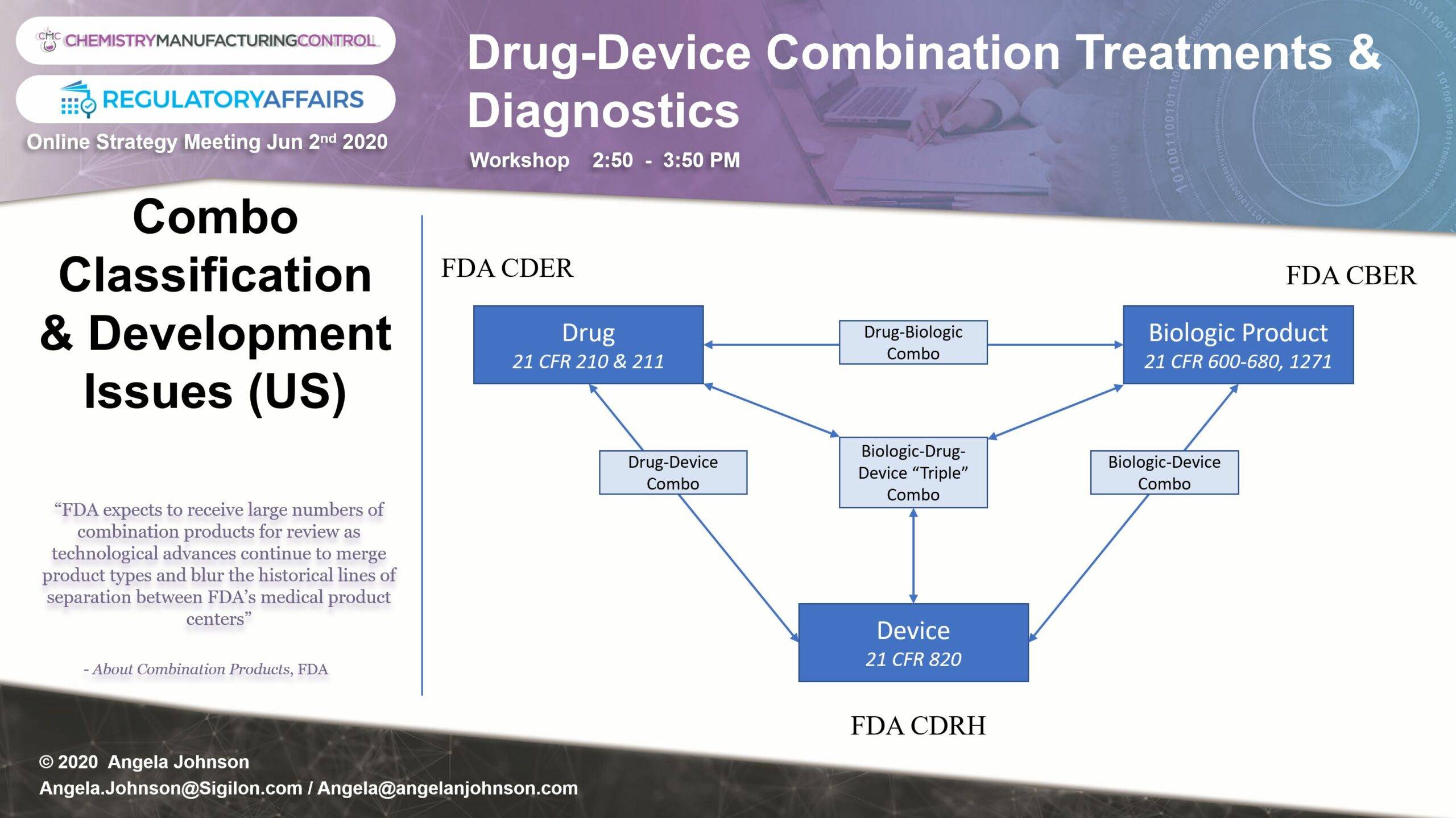
On June 2, 2020 as part of the Annual Online Chemistry, Manufacturing & Controls (CMC) Regulatory Affairs Series, sponsored by Proventa International, we joined chemistry, manufacturing, and controls (CMC) and regulatory affairs professionals from all over the world to engage with Senior peers in a round table format. The goals of the session were to:
- Explore the dynamic landscape for global classification and pathway selection for drug-, device-, and biologics-led combo products.
- Optimize your Companion Diagnostic strategy during drug and biologics development.
- Assess impact on drug development of new technologies in digital therapeutics, connected devices, and advanced materials.